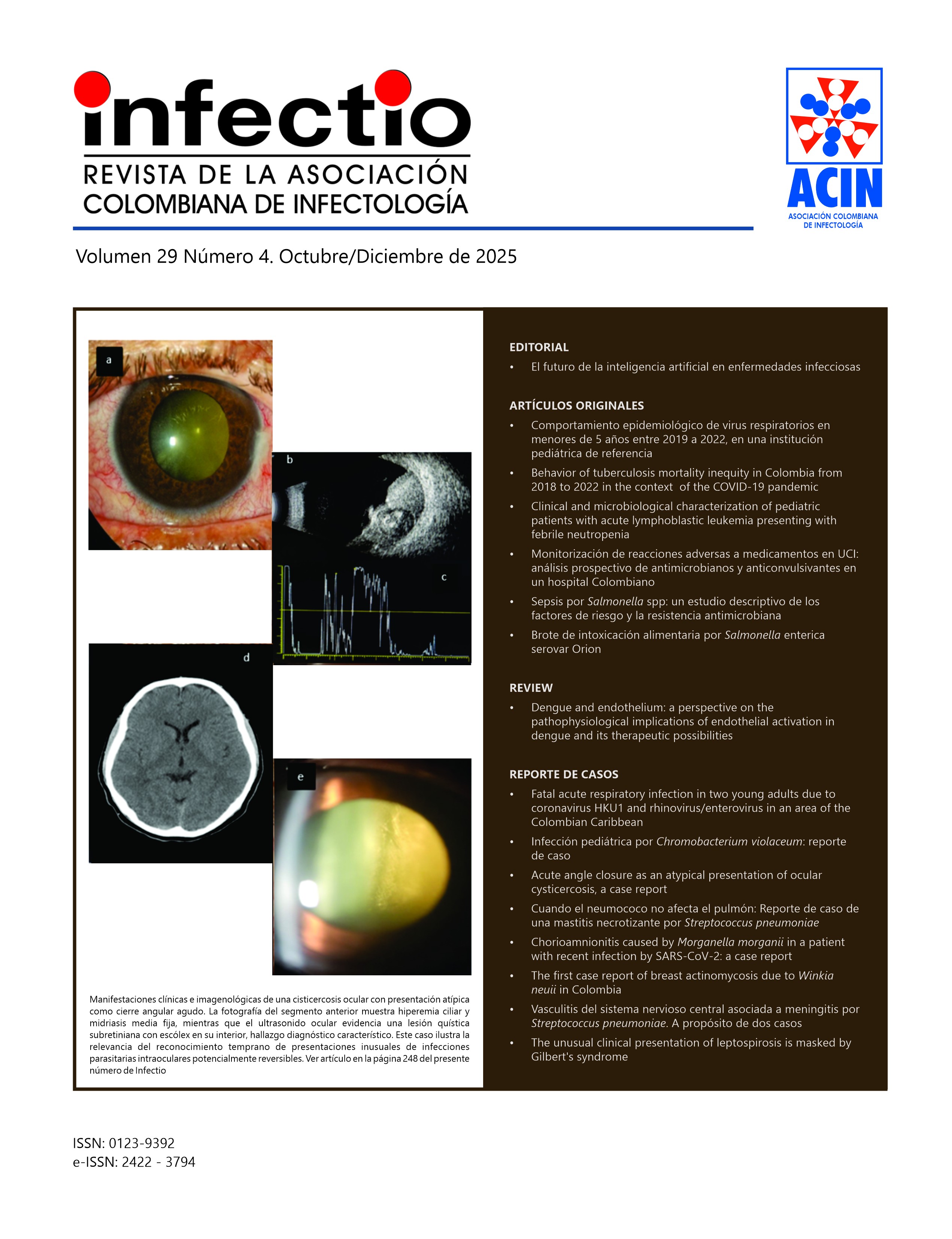
La Revista “INFECTIO” es el órgano oficial de divulgación de la Asociación Colombiana de Infectología - ACIN, se encuentra indexada en Scielo, Flying Publisher, Publimdex, Imbiomed, Doaj y Lilacs. Completa su décima edición y su circulación es trimestral. Publica artículos originales de investigación, revisiones y comentarios que tienen que ver con el área de las enfermedades infecciosas y ciencias afines.