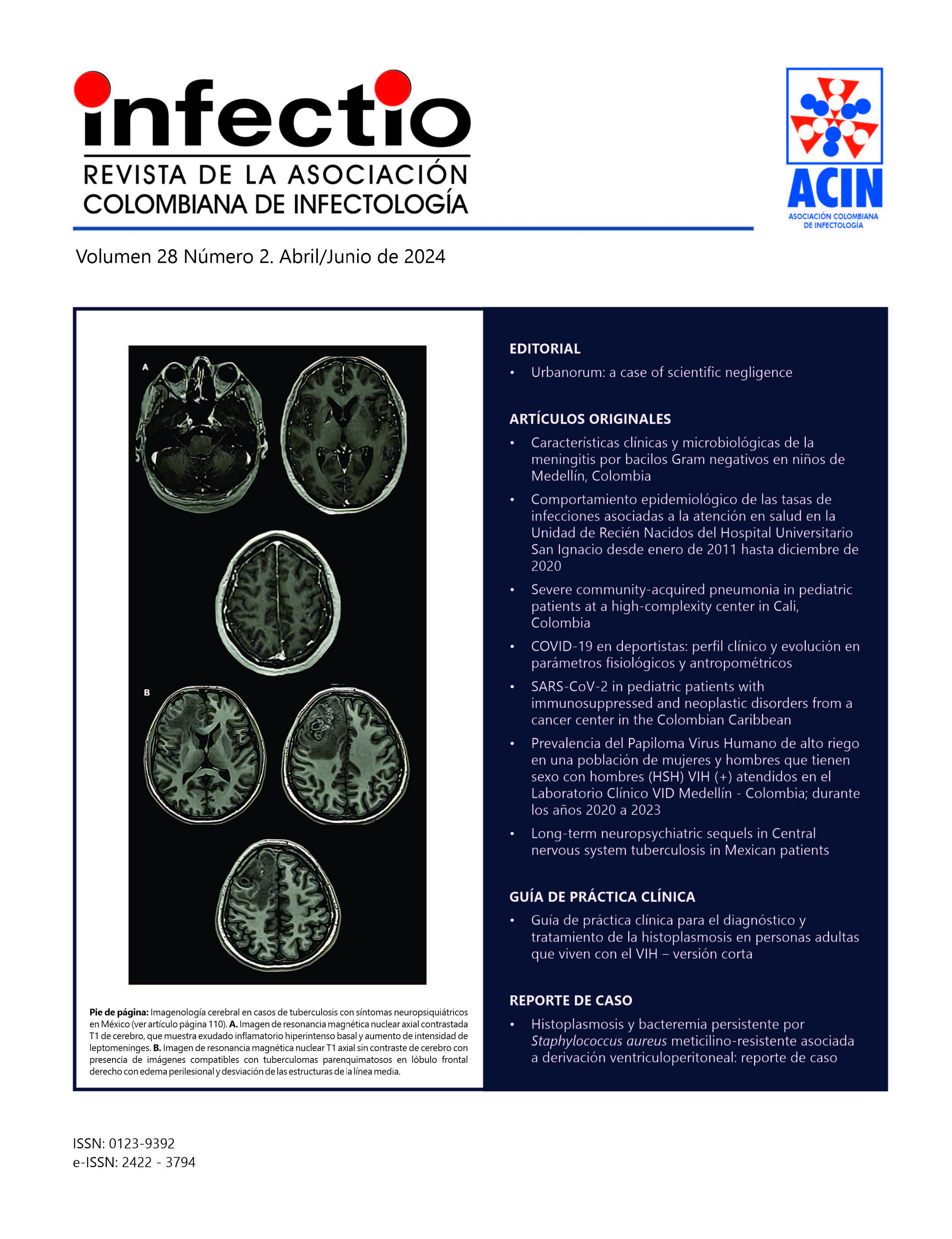
Secuelas neuropsiquiátricas a largo plazo en la tuberculosis del sistema nervioso central en pacientes mexicanos
Contenido principal del artículo
Resumen
Introducción: La tuberculosis del SNC (TB-SNC), es la forma más seria de TB, usualmente asociada a una respuesta neuroinflamatoria intensa y secuelas severas, además frecuentemente se acompaña de coinfección por virus de inmunodeficiencia humana (VIH). Pocos estudios han estudiado las secuelas mentales funcionales en esta población. Objetivo: Caracterizar las secuelas neuropsiquiátricas a largo plazo en pacientes con TB-SNC y en pacientes con la coinfección VIH/ TB-SNC, describiendo los factores asociados. Métodos: Estudio de cohorte retrospectiva en los pacientes con TB-SNC admitidos en un centro neurológico mexicano durante los años 2008 a 2018. Se colectaron los datos sociodemográficos, clínicos, de neuroimagen, cognitivos u neuropsiquiátricos Las secuelas cognitivas fueron obtenidas por herramientas normalizadas y estandarizadas para la población hispánica. Los datos neuropsiquiátricos a través del Cuestionario del Inventario neuropsiquiátrico y de los expedientes clínicos. Resultados: Se incluyeron un total de 86 sujetos, 23 tenían coinfección VIH/ TB-SNC. La edad promedio fue 40.4 años y 23% de ellos tenían historia de TB pulmonar. Los síntomas clínicos principales eran cefalea, fiebre y parálisis de nervios craneales. A nivel neurocognitivo, las secuelas más comunes implicaron afección de funciones ejecutivas, visuoespaciales y de memoria, mientras que las manifestaciones neuropsiquiátricas más frecuentes fueron depresión, irritabilidad y ansiedad. Se observe correlación entre la inmunidad (conteo de linfocitos T CD4+) y la afección de funciones ejecutivas. Conclusiones: Este es el primer trabajo evaluando las secuelas neurocognitivas a largo plazo en pacientes con TB en población mexicana. Algunos rasgos clínicos y sociodemográficos parecen ser factores neuroprotectores frente a secuelas neuropsiquiátricas a largo plazo.
Detalles del artículo
Citas
Chen HL, Lu CH, Chang CD, et al. Structural deficits and cognitive impairment in tuberculous meningitis. BMC Infect Dis. 2015;15:279. doi: https://doi.org/10.1186/s12879-015-1011-z
Kalita J, Misra UK, Ranjan P. Predictors of long-term neurological sequels of tuberculous meningitis: a multivariate analysis. Eur J Neurol. 2007; 14: 33-37. doi: https://doi.org/10.1111/j.1468-1331.2006.01534.x
Girard TD, Self WH, Edwards KM, et al. Long-Term Cognitive Impairment after Hospitalization for Community-Acquired Pneumonia: a Prospective Cohort Study. J Gen Intern Med. 2018;33:929-935. doi: https://doi.org/10.1007/s11606-017-4301-x
Calsavara AJC, Nobre V, Barichello T, et al. Post-sepsis cognitive impairment and associated risk factors: A systematic review. Aust Crit Care. 2018 ; 31 : 242-253. doi: https://doi.org/10.1016/j.aucc.2017.06.001
Muzambi R, Bhaskaran K, Brayne C, et al. Common bacterial infections and risk of incident cognitive decline or dementia: a systematic review protocol.BMJ Open. 2019; 9: e030874. doi: https://doi.org/10.1136/bmjopen-2019-030874
Klein RS, Garber C, Howard N. Infectious immunity in the central nervous system and brain function. Nat Immunol. 2017;18:132-141. doi: https://doi.org/10.1038/ni.3656
Kumar V. Tolll ke receptors in the pathogenesis of neuroinflammation. J Neuroimmunol. 2019; 332:16-30. doi: https://doi.org/10.1016/j.jneuroim.2019.03.012
Gale SD, Erickson LD, Brown BL, et al. Interaction between Helicobacter pylori and latent toxoplasmosis and demographic variables on cognitive function in young to middle-aged adults. PLoS One. 2015;10:e0116874. doi: https://doi.org/10.1371/journal.pone.0116874
Marais S, Thwaites G, Schoeman JF, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10:803-812. doi: https://doi.org/10.1016/S1473-3099(10)70138-9
Rock RB, Olin M, Baker CA, et al. Central nervous system tuberculosis: pathogenesis and clinical aspects. Clin Microbiol Rev. 2008; 21:243-261. doi: https://doi.org/10.1128/CMR.00042-07
Aguilar Navarro SG, Mimenza Alvarado AJ, Palacios García AA, et al. Validity and Reliability of the Spanish Version of the Montreal Cognitive Assessment (MoCA) for the Detection of Cognitive Impairment in Mexico. Rev Colomb Psiquiatr. 2018;47:237-243. doi: https://doi.org/10.1016/j.rcp.2017.05.003
Mallo SC, Patten SB, Ismail Z, et al. Does the neuropsychiatric inventory predict progression from mild cognitive impairment to dementia? A systematic review and meta-analysis. Ageing Res Rev. 2020; 58:101004. doi: https://doi.org/10.1016/j.arr.2019.101004
Musa G, Henríquez F, Muñoz Neira C, et al. Utility of the Neuropsychiatric Inventory Questionnaire (NPI-Q) in the assessment of a sample of patients with Alzheimer's disease in Chile. Dement Neuropsychol. 2017;11(2):129- 136. doi: https://doi.org/10.1590/1980-57642016dn11-020005
Ostrosky Solís F, Ardila A, Roselli, M. NEUROPSI: A brief neuropsychological test battery in Spanish with norms by age and educational level. J Int. 1999; 5: 413-433. doi: https://doi.org/10.1017/S1355617799555045
Warren SL, Heller W, Miller GA. The structure of executive dysfunction in depression and anxiety. J Affect Disord. 2021; 279:208-216. doi: https://doi.org/10.1016/j.jad.2020.09.132
DeBattista C. Executive dysfunction in major depressive disorder. Expert Rev Neurother. 2005;5(1):79-83 https://doi.org/10.1586/14737175.5.1.79
Sáenz B, Hernandez-Pando R, Fragoso G, et al. The dual face of central nervous system tuberculosis: a new Janus Bifrons? Tuberculosis. 2013;93:130-135. doi: https://doi.org/10.1016/j.tube.2012.11.011
Anderson NE, Somaratne J, Mason DF, et al. Neurological and systemic complications of tuberculous meningitis and its treatment at Auckland City Hospital, New Zealand. J Clin Neurosci. 2010;17:1114-1118. doi: https://doi.org/10.1016/j.jocn.2010.01.006
Ranjan P, Kalita J, Misra UK. Serial study of clinical and CT changes in tuberculous meningitis. Neuroradiology. 2003;45:277-282. doi: https://doi.org/10.1007/s00234-003-0958-4
Lin WC, Chen PC, Wang HC, et al. Diffusion tensor imaging study of white matter damage in chronic meningitis. PLoS One. 2014;9:e98210. doi: https://doi.org/10.1371/journal.pone.0098210
Duko B, Bedaso A, Ayano G. The prevalence of depression among patients with tuberculosis: a systematic review and meta-analysis. Ann Gen Psychiatry. 2020;19:30. doi: https://doi.org/10.1186/s12991-020-00281-8
Henri-Bhargava A, Stuss DT, Freedman M. Clinical Assessment of Prefrontal Lobe Functions. Continuum (Minneap Minn). 2018;24 (3) BEHAVIORAL NEUROLOGY AND PSYCHIATRY):704-726. doi: https://doi.org/10.1212/CON.0000000000000609
Guo Y, Schmitz TW, Mur M, et al. A supramodal role of the basal ganglia in memory and motor inhibition: Meta-analytic evidence. Neuropsychologia. 2018; 108:117-134. doi: https://doi.org/10.1016/j.neuropsychologia.2017.11.033
Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007; 362:917-932. doi: https://doi.org/10.1098/rstb.2007.2097
Davis AG, Rohlwink UK, Proust A, et al. The pathogenesis of tuberculous meningitis. J Leukoc Biol. 2019; 105:267-280. doi: https://doi.org/10.1002/JLB.MR0318-102R
Tai MLS, Viswanathan S, Rahmat K, et al. Cerebral infarction pattern in tuberculous meningitis. Sci Rep. 2016;6:38802. doi: https://doi.org/10.1038/srep38802
Takehara-Nishiuchi K. Entorhinal cortex and consolidated memory. Neurosci Res. 2014;84:27-33. doi: https://doi.org/10.1016/j.neures.2014.02.012
Hestad KA, Chinyama J, Anitha MJ, et al. Cognitive Impairment in Zambians With HIV Infection and Pulmonary Tuberculosis. J Acquir Immune Defic Syndr. 2019;80:110-117. doi: https://doi.org/10.1097/QAI.0000000000001880
Fernandez-Del Olmo A, Cruz-Cortes M, Conde C, et al. The role of the cognitive reserve in the cognitive recovery of patients who have suffered a severe addiction to substances. Rev Neurol. 2019; 69: 323-331. doi: 10.333588/rn.6908.2019095
Heaton RK, Franklin DR Jr, Deutsch R, et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis. 2015;60(3):473-480. doi: https://doi.org/10.1093/cid/ciu862
Kabuba N, Menon JA, Franklin DR, et al. Effect of age and level of education on neurocognitive impairment in HIV positive Zambian adults. Neuropsychology. 2018; 32: 519-528. doi: https://doi.org/10.1037/neu0000438
García Grimshaw M, Gutiérrez Manjarrez FA, Navarro Álvarez S, et al. Clinical, Imaging, and Laboratory Characteristics of Adult Mexican Patients with Tuberculous Meningitis: A Retrospective Cohort Study. J Epidemiol Glob Health. 2020; 10: 59-64. doi: https://doi.org/10.2991/jegh.k.191023.001
Mugusi S, Ngaimisi E, Janabi M, et al. Neuropsychiatric manifestations among HIV-1 infected African patients receiving efavirenz-based cART with or without tuberculosis treatment containing rifampicin. Eur J Clin Pharmacol. 2018; 74: 1405-1415. doi: https://doi.org/10.1007/s00228-018-2499-0
Smail RC, Brew BJ. HIV associated neurocognitive disorder. Handb Clin Neurol. 2018; 152:75-97. doi: https://doi.org/10.1016/B978-0-444-63849-6.00007-4
John CC, Carabin H, Montano SM, et al. Global research priorities for infections that affect the nervous system. Nature. 2015; 527: S178-S186. doi: https://doi.org/10.1038/nature16033
American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-V. 5th. Ed. Washington, D.C.: American Psychiatric Association; 2013. doi: https://doi.org/10.1176/appi.books.9780890425596
Duko B, Gebeyehu A, Ayano G. Prevalence and correlates of depression and anxiety among patients with tuberculosis at WolaitaSodo University Hospital and Sodo Health Center, WolaitaSodo, South Ethiopia, Cross sectional study. BMC Psychiatry. 2015;15:214. doi: https://doi.org/10.1186/s12888-015-0598-3
Chandra M, Rana P, Chandra K, et al. Tuberculosis - Depression syndemic: A public health challenge. Indian J Tuberc. 2019; 66:197-202. doi: https://doi.org/10.1016/j.ijtb.2019.02.007
Cavanna A. Motion and Emotion: The Neuropsychiatry of Movement Disorders and Epilepsy. 1st. Ed. New York: Springer International Publisher; 2018. doi: https://doi.org/10.1007/978-3-319-89330-3
Rosenberg PB, Nowrangi MA, Lyketsos CG. Neuropsychiatric symptoms in Alzheimer's disease: What might be associated brain circuits? Mol Aspects Med. 2015;43-44:25-37. doi: https://doi.org/10.1016/j.mam.2015.05.005
Giacobbe P, Flint A. Diagnosis and Management of Anxiety Disorders. Continuum (Minneap Minn). 2018;24(3, BEHAVIORAL NEUROLOGY AND PSYCHIATRY):893-919. doi: https://doi.org/10.1212/CON.0000000000000607
Deribew A, Deribe K, Reda AA, et al. Do common mental disorders decline over time in TB/HIV co-infected and HIV patients without TB who are on antiretroviral treatment? BMC Psychiatry. 2013;13:174. doi: https://doi.org/10.1186/1471-244X-13-174
Zhang K, Wang X, Tu J, et al. The interplay between depression and tuberculosis. J Leukoc Biol. 2019;106(3):749-757. doi: https://doi.org/10.1002/JLB.MR0119-023R
Khandaker GM, Dantzer R, Jones PB. Immunopsychiatry: important facts. Psychol Med. 2017;47:2229-2237. doi: https://doi.org/10.1017/S0033291717000745
Pape K, Tamouza R, Leboyer M, et al. Immunoneuropsychiatry - novel perspectives on brain disorders. Nat Rev Neurol. 2019;15:317-328. doi: https://doi.org/10.1038/s41582-019-0174-4
Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18:206-214. doi: https://doi.org/10.1038/mp.2012.110
López González I, Pinacho R, Vila E, et al. Neuroinflammation in the dorsolateral prefrontal cortex in elderly chronic schizophrenia. Eur Neuropsychopharmacol. 2019;29:384-396. doi: https://doi.org/10.1016/j.euroneuro.2018.12.011